Entrance Questions
Community chat: https://t.me/hamster_kombat_chat_2
Website: https://hamster.network
Twitter: x.com/hamster_kombat
YouTube: https://www.youtube.com/@HamsterKombat_Official
Bot: https://t.me/hamster_kombat_bot
Last updated 11 months, 2 weeks ago
Your easy, fun crypto trading app for buying and trading any crypto on the market.
📱 App: @Blum
🤖 Trading Bot: @BlumCryptoTradingBot
🆘 Help: @BlumSupport
💬 Chat: @BlumCrypto_Chat
Last updated 1 year, 4 months ago
Turn your endless taps into a financial tool.
Join @tapswap_bot
Collaboration - @taping_Guru
Last updated 12 months ago
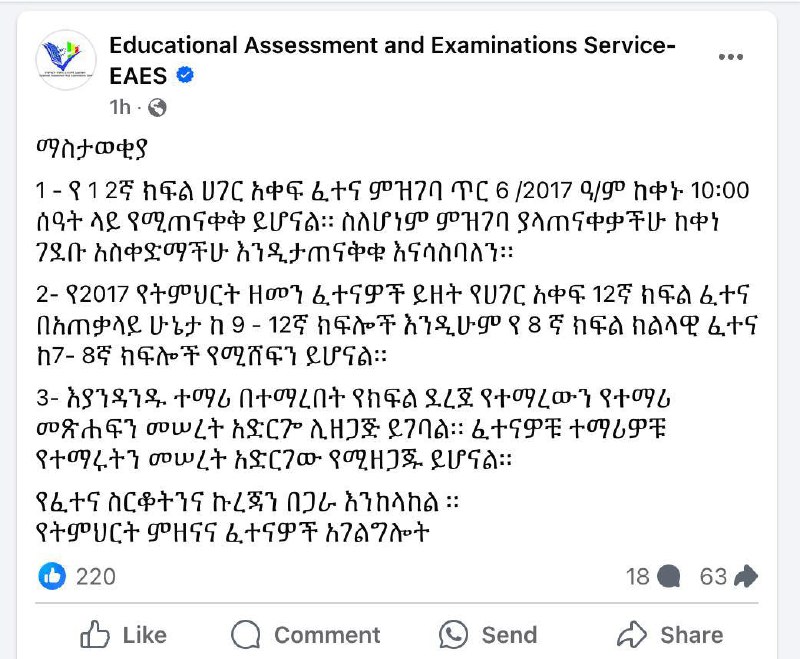
ዛሬ ት/ት ሚኒስቴር በፌስቡክ ገጹ ላይ በጻፈው ጽሁፍ ፤ የዘንድሮ ኢንትራንስ ፈተና ላይ የ10ኛ ክፍል አንደሚኖር አመላክቷል::
የ12ኛ ክፍል ሀገር አቀፍ ፈተና ምዝገባ ጥር 6/2017 ዓ.ም ከቀኑ 10፡00 ሰዓት ላይ እንደሚጠናቀቅ የትምህርት ምዘናና ፈተናዎች አገልግሎት አሳውቋል።
ስለሆነም ምዝገባ ያላጠናቀቃችሁ ተማሪዎች ከቀነ ገደቡ አስቀድማችሁ እንድታጠናቅቁ አገልግሎቱ አሳስቧል።
አገልግሎቱ የ2017 ዓ.ም ፈተናዎች ይዘትን በተመለከተ ባስተላለፈው መልዕክት፤ የሀገር አቀፍ 12ኛ ክፍል ፈተና በአጠቃላይ ከ 9-12ኛ ክፍሎች እንዲሁም የ8ኛ ክፍል ክልላዊ ፈተና ከ7-8ኛ ክፍሎች እንደሚሸፍን ገልጿል።
ተማሪዎች በተማሩበት የክፍል ደረጀ የተማሪ መጽሐፍን መሠረት አድርገው ሊዘጋጁ እንደሚገባ አገልግሎቱ ጠቁሟል።
የትምህርት ምዘናና ፈተናዎች አገልግሎት
Short Note
Unit 1: Atomic Structure and Periodic Properties of the Elements
- Atomic Structure: Atoms consist of a nucleus containing protons (positively charged) and neutrons (neutral), surrounded by electrons (negatively charged) in defined energy levels or shells. The arrangement of electrons determines an element's chemical properties.
- Electron Configuration: Electrons occupy orbitals in a specific order, following the Aufbau principle, which states that electrons fill lower-energy orbitals first. This configuration influences how elements interact chemically.
- Periodic Table: Elements are arranged in the periodic table by increasing atomic number, with rows (periods) indicating the number of electron shells and columns (groups) indicating elements with similar valence electron configurations. This arrangement reflects periodic trends in properties such as electronegativity, ionization energy, and atomic radius.
- Isotopes: Atoms of the same element can have different numbers of neutrons, resulting in isotopes with varying mass numbers but identical chemical properties.
Unit 2: Chemical Bonding
- Ionic Bonds: Formed when electrons are transferred from one atom to another, resulting in oppositely charged ions that attract each other. This type of bonding typically occurs between metals and non-metals.
- Covalent Bonds: Involve the sharing of electron pairs between atoms, allowing each to attain the electron configuration of a noble gas. Covalent bonding usually occurs between non-metal atoms.
- Metallic Bonds: In metals, atoms share a "sea" of delocalized electrons, which allows for properties like electrical conductivity and malleability.
- Intermolecular Forces: Weaker than chemical bonds, these forces (such as hydrogen bonds, dipole-dipole interactions, and London dispersion forces) influence physical properties like boiling and melting points.
Join our Educational Channels:
▬▬▬▬▬▬▬▬▬▬▬▬▬
?JOIN: @Educational_Question
?JOIN: @Et_Study_Notes
?JOIN: @Oromia_Educational_News
?JOIN: @AmboIfaBoru
?JOIN: @General_questions_always
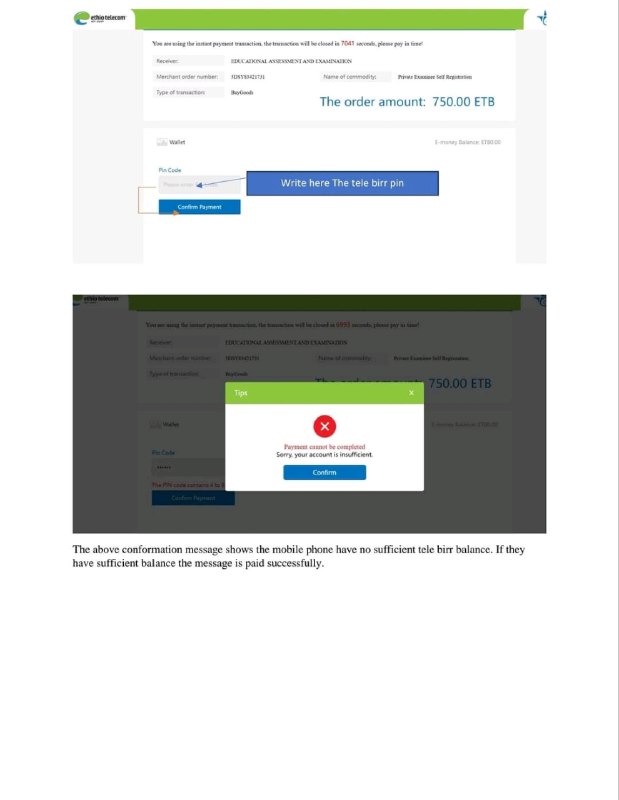
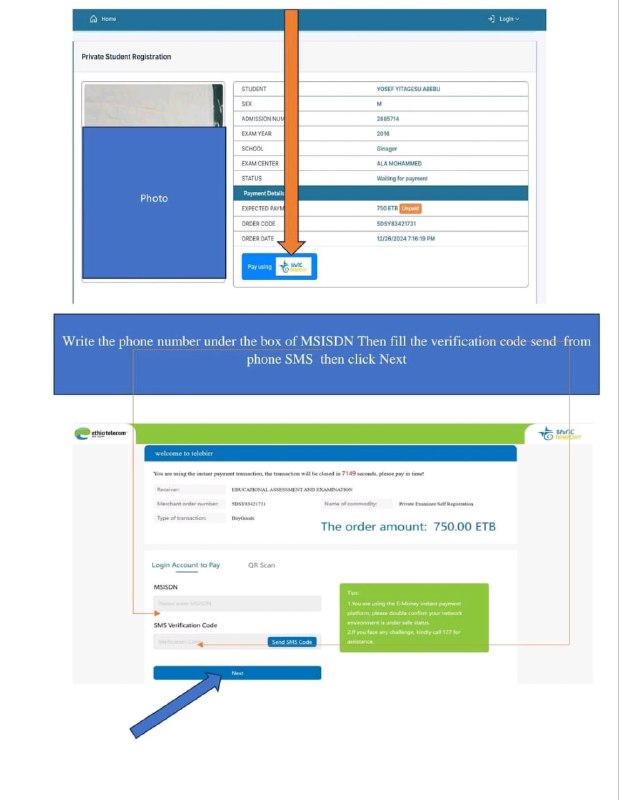
checkout new resources ???
https://t.me/Et_Study_Notes
https://t.me/Et_Study_Notes
Community chat: https://t.me/hamster_kombat_chat_2
Website: https://hamster.network
Twitter: x.com/hamster_kombat
YouTube: https://www.youtube.com/@HamsterKombat_Official
Bot: https://t.me/hamster_kombat_bot
Last updated 11 months, 2 weeks ago
Your easy, fun crypto trading app for buying and trading any crypto on the market.
📱 App: @Blum
🤖 Trading Bot: @BlumCryptoTradingBot
🆘 Help: @BlumSupport
💬 Chat: @BlumCrypto_Chat
Last updated 1 year, 4 months ago
Turn your endless taps into a financial tool.
Join @tapswap_bot
Collaboration - @taping_Guru
Last updated 12 months ago